Atomic Model
Atomic Model: Overview
This topic covers concepts, such as, Atomic Model, The Thomson Model, Calculation of Specific Charge by Thomson's Method & Explanation of Emission of Electromagnetic Radiations from Atom Using Thomson's Model of Atom etc.
Important Questions on Atomic Model
In Rutherford scattering experiment, the correct angle of scattering of α− particles for impact parameter equal to zero is
In Rutherford's experiment, the alpha particles that come closer to the nuclei are
Out of the following statements regarding Rutherford's model, which of the following is/are correct?
a. Most of the space inside an atom is empty.
b. The electrons revolve around the nucleus under the influence of coulomb force acting on them.
c. Most part of the mass of the atom and its positive charge are concentrated at its centre.
d. The stability of atom was established by the model.
Velocity of electron orbiting around nucleus of hydrogen atom is proportional to radius (r)
Radius of the smallest orbit of hydrogen atom is
The gravitational force between a -atom and another particle of mass will be given by Newton's law , where is in metre and
Geiger-Marsden experiment is related with the size of the-
A beam of fast moving alpha particles were directed towards a thin film of Gold. The parts and of the transmitted and reflected beams corresponding to the incident parts and of the beam are shown in the adjoing diagram. The number of alpha particles in
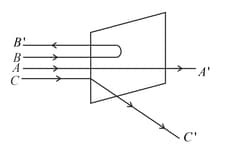
On which of the following factors does the trajectory of an alpha particle depend?
The significant result deduced from the Rutherford's scattering experiment is that
Rutherford -scattering demonstrates the existence of which of the following ?
What is the conclusion of Rutherford’s alpha particle scattering experiment ?
An atom is electrically neutral because there are
The number of completely filled shells for the element is
Particle used in the Rutherford's scattering experiment to deduce the structure of atoms
Consider the following statement:
(I) All isotopes of elements have the same number of neutrons.
(II) Only one isotope of an element can be stable and non-radioactive.
(III) All elements have isotopes.
(IV) All isotopes of Carbon can form chemical compounds with Oxygen.
The correct option regarding an isotope is:
In 1911, the physical Ernest Rutherford discovered that atoms have a tiny, dense nucleus by shooting positively charged particles at a very thin gold foil. A key physical property which led Rutherford to use gold was that it was
Three particles having charges in the ratio of produce the same point on the photographic film in Thomson experiment. Their masses are in the ratio of
